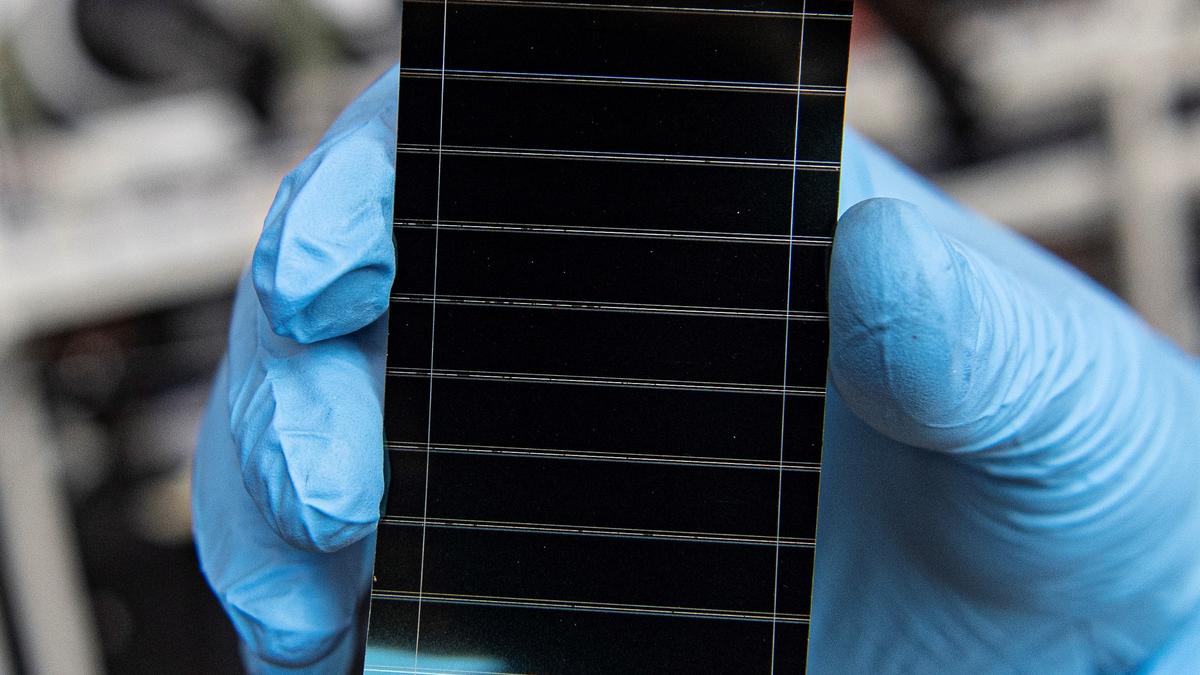
Using solar energy may be better for the environment than burning through fossil fuels, but the process still isn’t exactly perfect. Making silicon-based solar panels is energetically expensive and we still don’t know what to do with the silicon once the panels are done being used.
There is a cheaper way to make solar panels, also called photovoltaics (PVs), using crystal structures called perovskites. However, perovskite crystals contain toxic elements like lead, which needs to be processed carefully once these solar panels reach the end of their lifespan. And so far, researchers have had to use toxic organic solvents like dimethylformamide to recycle such solar panels.
A yummy sandwich
Now, in a paper published in Nature, scientists have described another, potentially greener way of dealing with the problem. Using a water-based recycling solution, they have reported a way to degrade and recycle used perovskite. They were also able to get back high-quality perovskite crystals, which can potentially be used again for making new solar cells.
“It’s kind of a complex chemistry to make the water solution usable and very stable for perovskite recycling, to fully remove the [the use of] organic solvents,” Xun Xiao, a postdoctoral researcher at Linköping University in Sweden and lead author of the paper, said.
Perovskite solar cells are made up of multiple layers. The perovskite layer is sandwiched between materials that can conduct and transport charges, in this case metal electrodes and glass sheets.
“People have been very excited about [perovskite PVs] for a decade or more now because very quickly they have been able to achieve very high power conversion efficiencies,” Rhys Charles, a researcher in the chemical engineering department at Swansea University in the UK explained. “So you could deliver an extremely cheap solar energy technology, but there are some things that have been holding the field back.”
Stability is one of them: perovskite solar cells have a shorter lifespan.
Improving solar energy
“Early attempts to recycle these devices have all focused on capturing lead. Now, people are taking a little bit more of a holistic view of it,” Charles continued. “From a circular economy point of view, recycling is also important because they want to capture the major impact materials [that] they use again. “
For a circular economy, the aim is to keep the product — in this case the components of a perovskite solar cell — in use for as long as possible, to minimise waste. This way, if the cells are made again with recycled components, they would have a much lower environmental impact, which means lower emissions and lower cost associated with solar energy generation.
Thus far, the only way to recycle these important materials has been to use toxic organic solvents.

Acids and salts
Dissolving and recycling the lead-containing perovskite layer in water was a major challenge to overcome. For this, the scientists added three key salts to help in the recycling process.
The first salt they added was sodium acetate. The acetate ions bound with the lead ions in the perovskite, making a highly soluble lead acetate that dissolved well in water.
They then added sodium iodide and hypophosphorous acid to help regenerate pure perovskite crystals in their water solution. Sodium iodide contains iodide ions that help repair and restore the degraded perovskite, such that when the solution is cooled, high-quality, pure perovskite crystals re-emerge from the solution.
The acid acts as a long-term stabiliser, ensuring the water solution can be reused and that the quality of the recycled crystals remains high.
‘Solved the problem’
“I’m pleased to see this focus on recycling, remanufacturing, and green chemistry,” said Matthew Davies, a professor of chemical engineering at Swansea University. “It lays the foundation for perovskite PVs to deliver on their promise as a low-cost, high-efficiency solar technology within a circular economy, avoiding the large-scale waste challenges faced by earlier PV technologies.”
The scientists also developed solutions made of ethanol and ethyl acetate to dissolve other components of the perovskite solar cell, after which they were able to recycle each component to reuse along with the perovskite crystals. Then they reassembled the solar cell layer by layer and found that the efficiency was almost the same as using fresh materials, even after being recycled up to five times. They were also able to re-obtain about 99% of the different layers even after multiple rounds of recycling.

“These guys seem to have solved the problem; they seem to be able to use this aqueous system to recycle the cells and make them again with high efficiency,” Charles said. “If it can be scaled [up] and if it works as well as the paper claims, it could potentially overcome some of the most significant barriers to commercialising perovskites and solve some of the key environmental concerns about the technologies as well.”
Charles also stressed the need to underpin scientific and industrial progress, especially when it came to environmental technologies, with life cycle assessments. Life cycle assessment, he explained, is an approach to quantify all the impacts of a technology across its entire life cycle, from the start to when one has the final product. “But you can go further,” he said. “Then you can look at the use phase of the technology and the end-of-life phase as well.”
“I always enjoy it when I see these things underpinned by life cycle assessment, to make sure there aren’t unintended consequences and to make sure the research really is targeting the key environmental problems for the technology,” Charles added. “I’d like to see more of that as well, as just standard practice.”
Rohini Subrahmanyam is a freelance journalist in Bengaluru.
Published – April 21, 2025 05:30 am IST